Helping Emergency Responders Obtain Support (HEROS) Program
Helping Emergency Responders Obtain Support (HEROS) is a program at the Department of Children and Families (DCF) that provides emergency responders with emergency opioid antagonist products free of charge.
Frequently Asked Questions
General Program Information
The HEROS Program is not a grant. Emergency opioid antagonist products obtained through this program are paid for by Florida’s Opioid Settlement funding.
There are multiple opportunities available for emergency opioid antagonist product administration training. Virtual sessions are conducted by a member of our team and include a live question-and-answer component.
To participate, external users must first complete the registration process outlined below. We strongly encourage all participants to create a MyFLLearn account, as this is required for the issuance of a training certificate upon completion.
If you wish to invite additional participants who do not have a MyFLLearn account, you may share the Microsoft Teams meeting link with them directly. Once your session has launched in MyFLLearn, you will be able to copy and share the meeting details with others from within the session interface.
Once you have registered, you will be able to access the Learning Events Calendar and choose which date you would like to attend.
New Account Registration
1. Register for an account HERE.
2. Follow the login steps to sign into MyFLLearn.
- Enter your email address as your “Username”.
- Enter the password you created when you signed up in the “Password” field.
- Click on “Manual Login”.
Additionally, self-paced online training is available through the approved source Learn How to Respond to an Overdose | serotarcnetwork.org
Eligibility Requirements
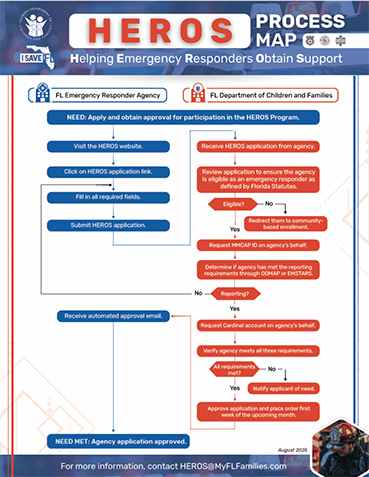
- All applicants must meet the definition of an emergency responder agency or have a written agreement to supply emergency responders with the product. An emergency responder agency is any organization that has staff who are trained to arrive to the scene of an emergency to provide immediate assistance or who work in a correctional capacity, whether in the community or at a facility. Emergency responder agencies include police, fire, sheriff, corrections, probation, EMS providers, as well as volunteer fire and ambulance services. Applicants must meet the emergency opioid antagonist administration reporting requirements:
- For law enforcement/non-licensed fire agencies: Applicants must register for the Washington/Baltimore High Intensity Drug Trafficking Overdose Detection Mapping Application Program (ODMAP) free of charge at https://www.odmap.org/AgencyAccess/RequestForm
- For Licensed EMS agencies: Applicants must report through the Emergency Medical Services Tracking and Reporting System (EMSTARS – Emergency Medical Services Tracking And Reporting System (floridaemstars.com)) Version 3.
- The Department will create a Minnesota Multi-State Contracting Alliance for Pharmacy Agreement (MMCAP) account on your agency’s behalf.
- Applicants must also agree to have DCF set-up an account with Cardinal Health, the product wholesaler, for shipping purposes. This ensures the billing information is accurate for Department of Children and Families payment.
No, it is not required at the time of initial application, however, all eligibility requirements must be met and verified by DCF before any emergency opioid antagonist product can be shipped. It is important to pursue the eligibility requirements as soon as possible.
Application Process
An agency contact notifies the HEROS Program team that their agency is interested in enrolling in the HEROS Program. The HEROS Program staff will obtain the MMCAP. Once the MMCAP is obtained, the HEROS Program staff will request an account from Cardinal Health, the wholesale distributor.
The agency is responsible for applying to Overdose Mapping Application Program (ODMAP) or EMSTARS based on the organization’s emergency responder status.
No, each agency must submit a separate request regardless of affiliation.
Reporting Requirement
ODMAP is a free tool provided by the High Intensity Drug Trafficking Areas (HIDTA) Program. ODMAP provides real-time overdose surveillance data across jurisdictions to support public safety and health efforts to mobilize an immediate response to an overdose spike. It is a tool that the Department of Children and Families uses to monitor and report emergency opioid antagonist administrations by emergency responders. Refer to the following link: at High Intensity Drug Trafficking Areas (hidta.org).
The timeline depends on how quickly the registration is processed within your agency, particularly regarding the electronic signing of the participation agreement. However, most agencies typically receive full access within 24 hours.
Yes, EMS agencies must report to EMSTARS or ODMAP to enroll with the HEROS Program.
Minnesota Multi-State Contracting Alliance for Pharmacy Agreement (MMCAP)
No, the Department of Children and Families works with MMCAP to ship the product directly to each enrolled agency. There is no charge to HEROS-enrolled agencies.
Yes, the products are purchased through MMCAP at volume pricing and supplied directly to the applicant through the HEROS program.
No, please provide the current MMCAP ID number in your HEROS application.
Yes, this is handled directly between your agency and MMCAP.
Ordering and Type of Product Available
Eligible agencies may order emergency opioid antagonist products monthly, as needed. All orders are processed the first week of the following month. The applicant must submit a new order/application for each request. If an agency runs low on emergency opioid antagonist products and additional doses are needed before the next monthly orders are placed, please reach out to HEROS@myflfamilies.com.
At this time, orders are submitted through the Health Operations and Medical Emergency Response (HOMER) System by creating a “new application”.
See below:
- Option # 1 Intranasal (naloxone HCL) (NDC # 69547-0627-02) – 4mg dose
- Option # 2 Luerlock pre-filled syringe (naloxone HCL) (NDC # 76329-3369-01) – 2ml
- Option # 3 MIN-I-JET 21ga x 1-1/2” syringe (naloxone HCL) (NDC # 76329-1469-01) – 2ml
- Option # 4 Vial (naloxone HCL) (NDC # 00641-6132-25) – 0.4mg/ml = 1 vial
- Option # 5 KLOXXADO (naloxone HCL) (NDC # 59467-0679-01) – 8 mg/0.1ml
- Option # 6 Auto-injector FOR PROPHYLACTIC USE (naloxone HCL) (NDC # 60842-0002-02) 10 mg/0.4ml
Yes. Indicate the number of doses being requested for each type of product on the application. For example: If you order 30 doses of Option #1, you will receive 15 boxes as each box contains two doses.
Each HEROS product shipment may have various expiration dates. It is recommended that each HEROS agency use the products within their agency’s inventory with the earliest expiration date first.
See below:
- Option # 1 Intranasal (naloxone HCL) (NDC # 69547-0627-02) – 4mg dose
- Option # 2 Luerlock pre-filled syringe (naloxone HCL) (NDC # 76329-3369-01) – 2ml
- Option # 3 MIN-I-JET 21ga x 1-1/2” syringe (naloxone HCL) (NDC # 76329-1469-01) – 2ml
- Option # 4 Vial (naloxone HCL) (NDC # 00641-6132-25) – 0.4mg/ml = 1 vial
- Option # 5 KLOXXADO (naloxone HCL) (NDC # 59467-0679-01) – 8 mg/0.1ml
- Option # 6 Auto-injector FOR PROPHYLACTIC USE (naloxone HCL) (NDC # 60842-0002-02) 10 mg/0.4ml
Note: Order the number of doses your agency needs.
Shipping and Receiving Product
Two to four weeks after the HEROS Team validates that a HEROS’ applicant’s eligibility requirements have been met. The Department of Children and Families will place an order with Cardinal Health once a month (first week of each month, based on availability of funding).
We will notify the day before the product is shipped.
This depends on what product is ordered and the current availability of the product(s).
Please have the totes from previous deliveries ready to give back to the delivery person.
Expired Product
Please contact the organization below to send the expired kit to:
Michelle Pepin at pickingupthepieces2020@gmail.com
(352) 816-3793
2843 SW 20th Street
#7
Ocala, FL
34474
OR
Caitlin Y Perez at caitlinnelson2012@yahoo.com
(813) 310-8622
We recommend checking your emergency opioid antagonist supply every few weeks. If you have products approaching their expiration date, please notify us promptly. This allows us to redirect them to a nearby distributor who can ensure they are distributed to individuals at risk in a timely manner. To minimize waste, consider placing smaller, more frequent orders to ensure products are used before expiration. Additional shipments can be requested as needed.
Expired products may still be used for demonstration purposes during training sessions only. Otherwise, please dispose of expired products appropriately or send to Michelle Pepin or Caitlin Perez listed above. The National Association of Boards of Pharmacy provides a helpful resource for locating drug disposal sites, available at Drug Disposal – Safe Pharmacy.